- License Req
8490 Astrazeneca - XYL1050
Pack Qty
Restricted Product
Unit of measure
EA
Quantity
Restricted item. To purchase, you will need to be a dentist or doctor with a valid GDC/GMC or equivalent licence.
Non-returnable unless faulty.
Description
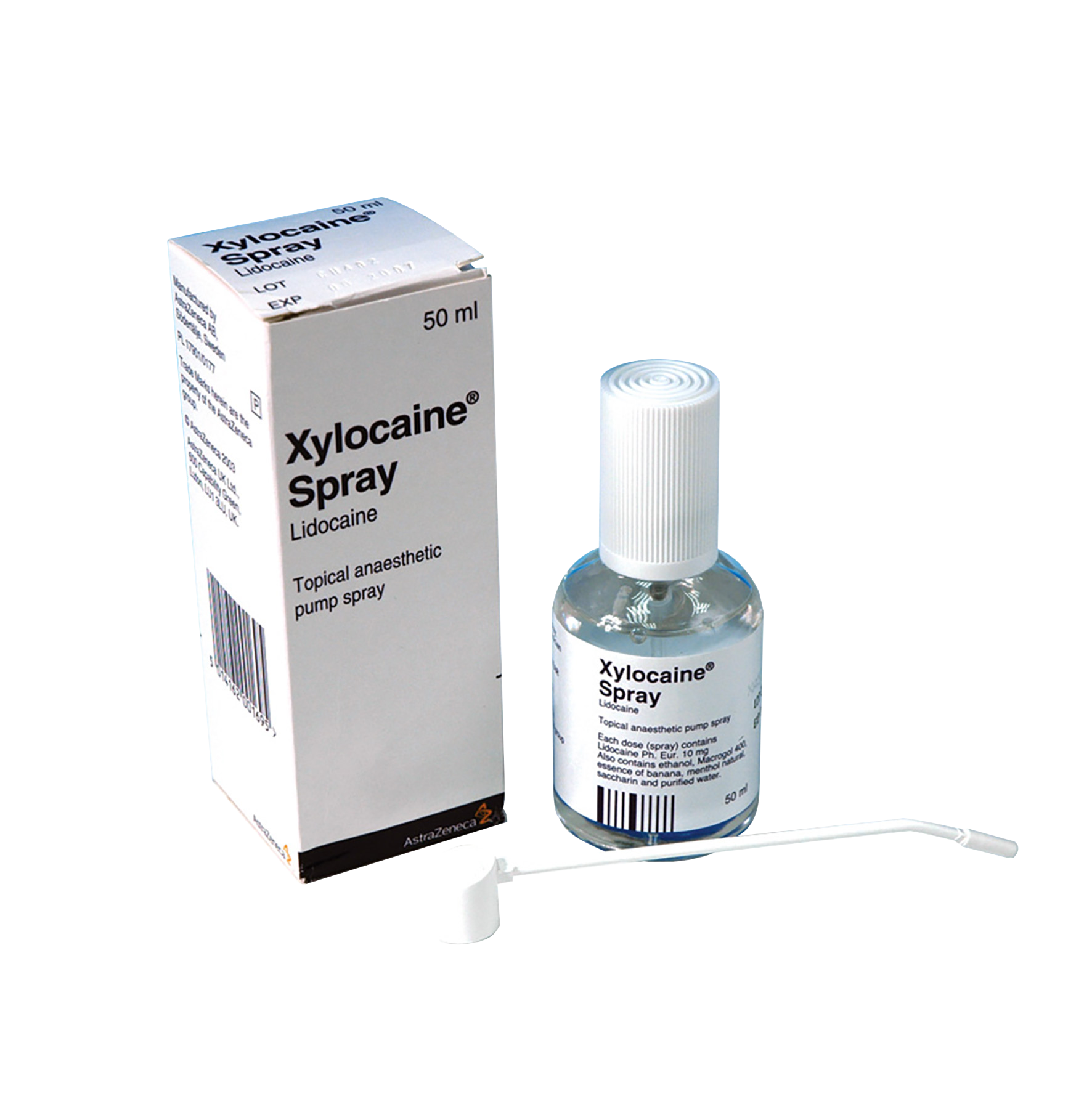
Topical spray anaesthetic. Each dose contains lidocaine Ph. Eur./10 mg. Also contains ethanol, polyethylene glycol 400, essence of banana, menthol, natural saccharin and purified water. Not licensed for sale in the Republic of Ireland.
Product Attributes
Category
KENTXPRS_S10109- Topical Spray & Gel
Additional Attributes
Return Policy
Download Documents
1001035_UK_MSDS_01_-Xylocaine-Topical-Anaesthetics-Spray-50ml.pdf
PDF
Specifications
Manufacturer
Astrazeneca
GDC/GMC
Required
Manufacturer Product Code
XYL1050
Pack Qty
1
Product Sub Type
Topical Spray & Gel
Product Type
Anaesthetics & Needles
)